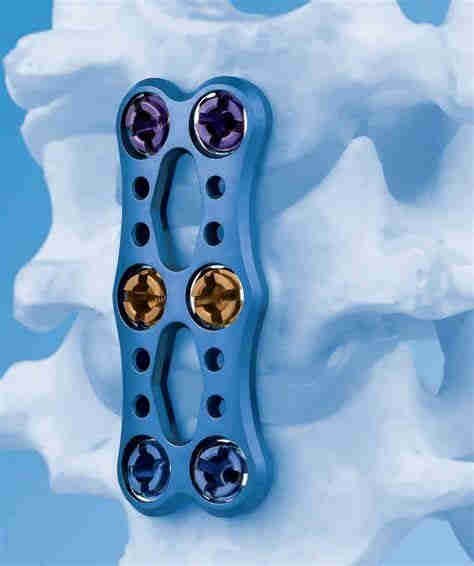
Product Line: VECTRA Plate
Description:
TI VECTRA(TM) PLATE 2 LEVEL/32MM
Bone-screw internal spinal fixation system, non-sterileCategory 1: Spine
Category 2: Cervical Plate & Screw Systems
Category 3: Plate
Category 4: Two Level
Quantity of "Each"es needed in each System/Set: 1
FDA Regulation Medical Specialty: Orthopedic Devices - Prosthetic Devices
FDA Regulation Number: 888.3060
FDA Regulation Description Classification Name: Spinal intervertebral body fixation orthosis
FDA Classification Product Code: KWQ
FDA Classification Product Code Device Name: Appliance, Fixation, Spinal Intervertebral Body